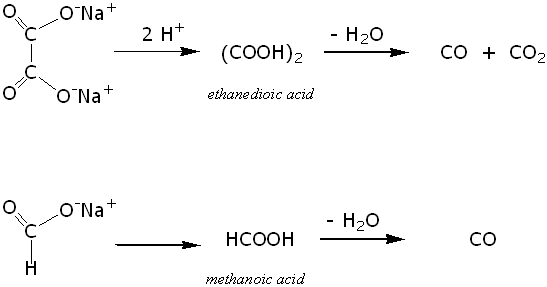
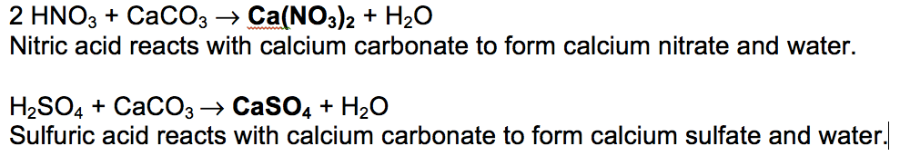Marble And Sulfuric Acid Reaction
Sulphuric acid is a compound it is not sulfur dioxide and water the formula is h2so4sulphuric acid is the product of the chemical reaction that occurs when sulfur trioxide and water are mixed.
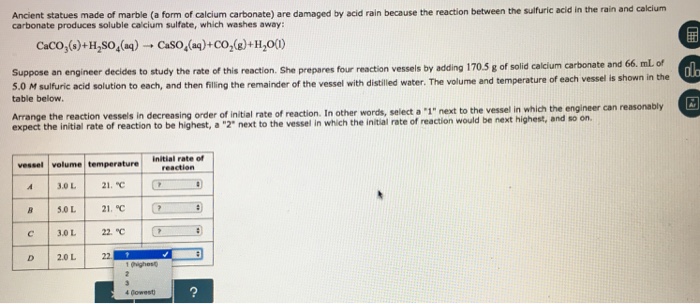
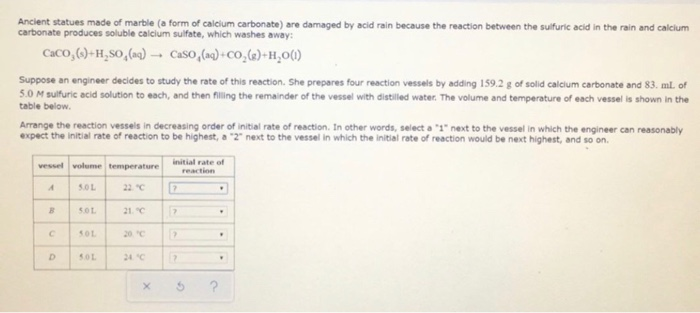
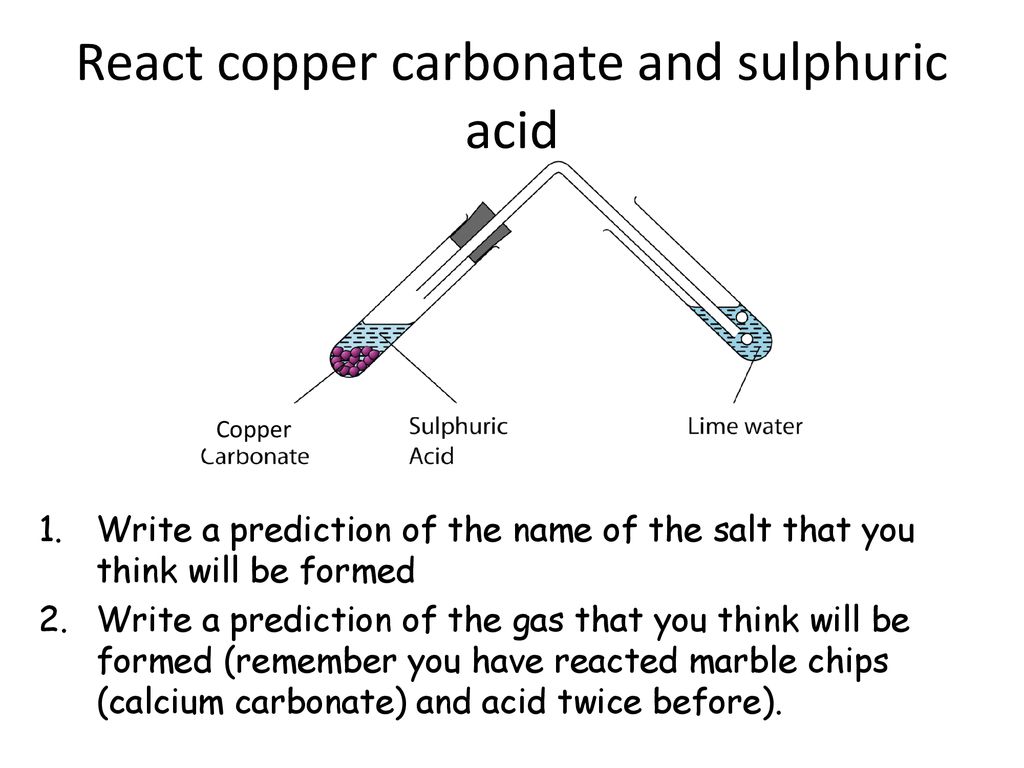
Marble and sulfuric acid reaction. Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas. Create a dehydration reaction using sugar and sulfuric acid. The balanced equation is. However sheltered areas on limestone and marble buildings and monuments show blackened crusts that have spalled peeled off in some places revealing crumbling stone beneath.
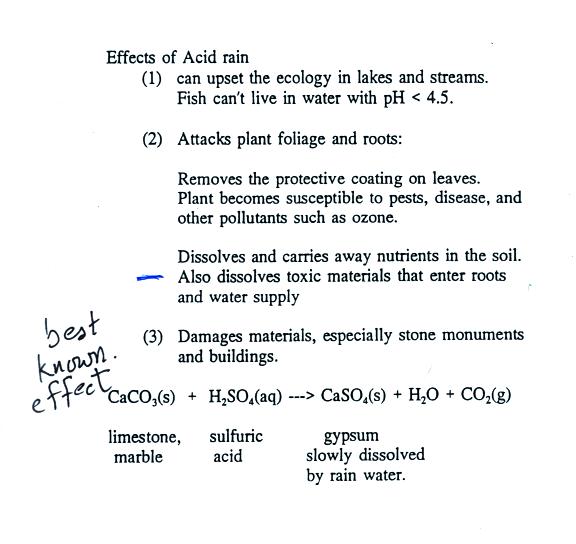
Stone surface material may be lost all over or only in spots that are more reactive. Thus the presence of h 2 so 4 causes the concentration of h ions to increase dramatically and so the ph of the rainwater drops to harmful levels. In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details. This black crust is primarily composed of gypsum a mineral that forms from the reaction between calcite water and sulfuric acid.
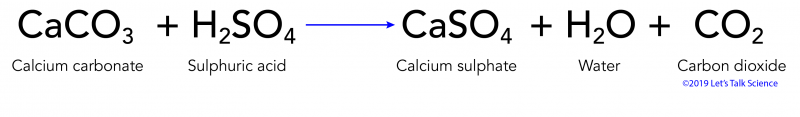
Caco3 2hno3 ca no3 2 h2o co2 g i believe this reaction happens regardless of the concentration of nitric acid since. H2co3 aq h2o l co2 g so caco3 s h2so4 l h2o l co2 g caso4 s so when calcium. When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves. Caco3 s h2so4 l h2co3 aq caso4 s 2.
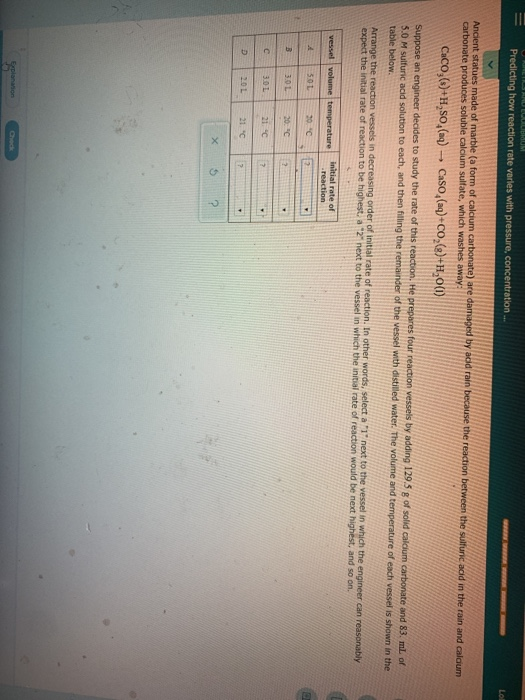
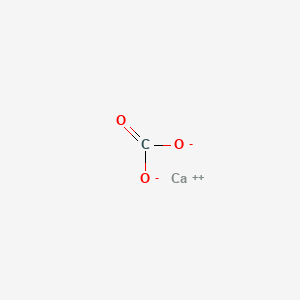
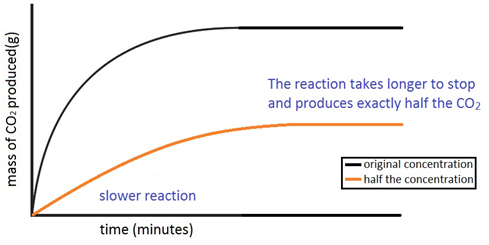
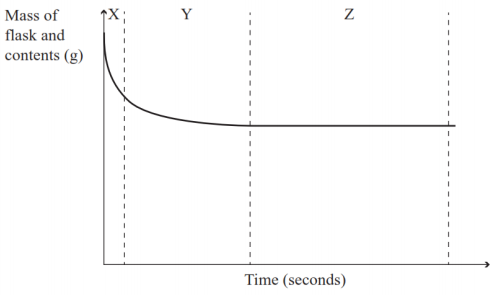
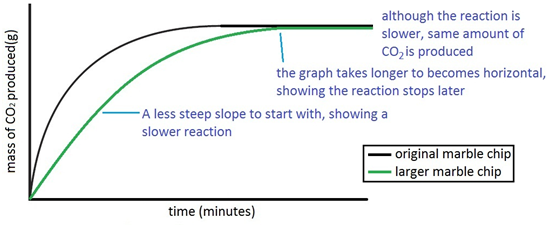
Dilute sulphuric acid is made to react with marble chips 2 see answers mahfoozfarhan4 mahfoozfarhan4 so when calcium carbonate reacts with sulphuric acid it forms water carbon dioxide and calcium sulphate. The hso 4 ion may further dissociate to give h and so 4 2 equation 8. The rate of this reaction can be changed by changing the size of the marble chips. When acids react with carbonates such as calcium carbonate found in chalk limestone and marble a salt water and carbon dioxide are made.
The reaction between sulfuric acid and calcium carbonate is somewhat similar to the reaction with sodium bicarbonate way carbon dioxide.