Marble Chips And Hydrochloric Acid
Marble chips and hydrochloric acid planning aim to find if changing the concentration of an acid will increase or decrease the rate of the reaction when marble is dissolved in hydrochloric acid.
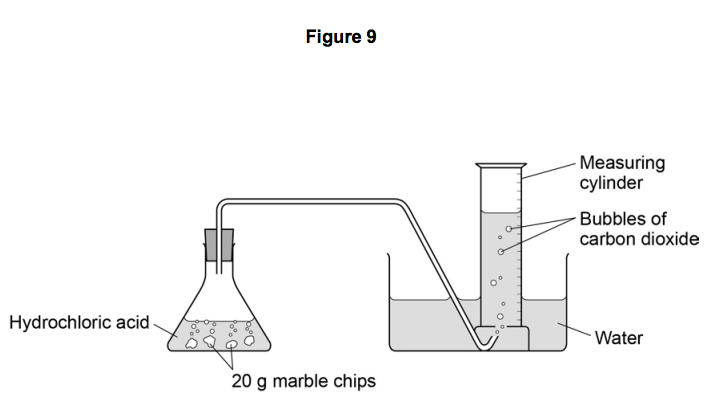
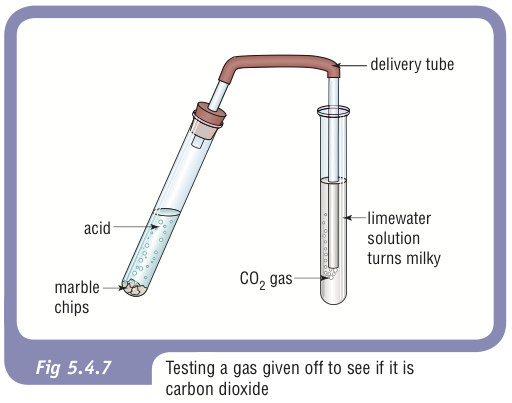
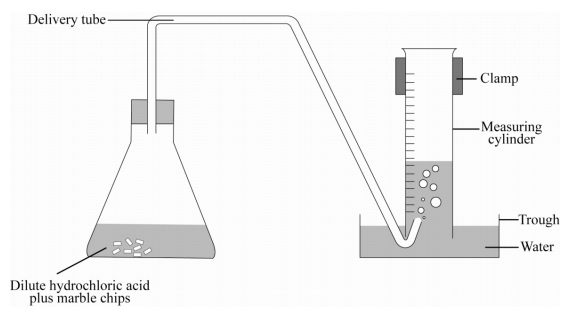
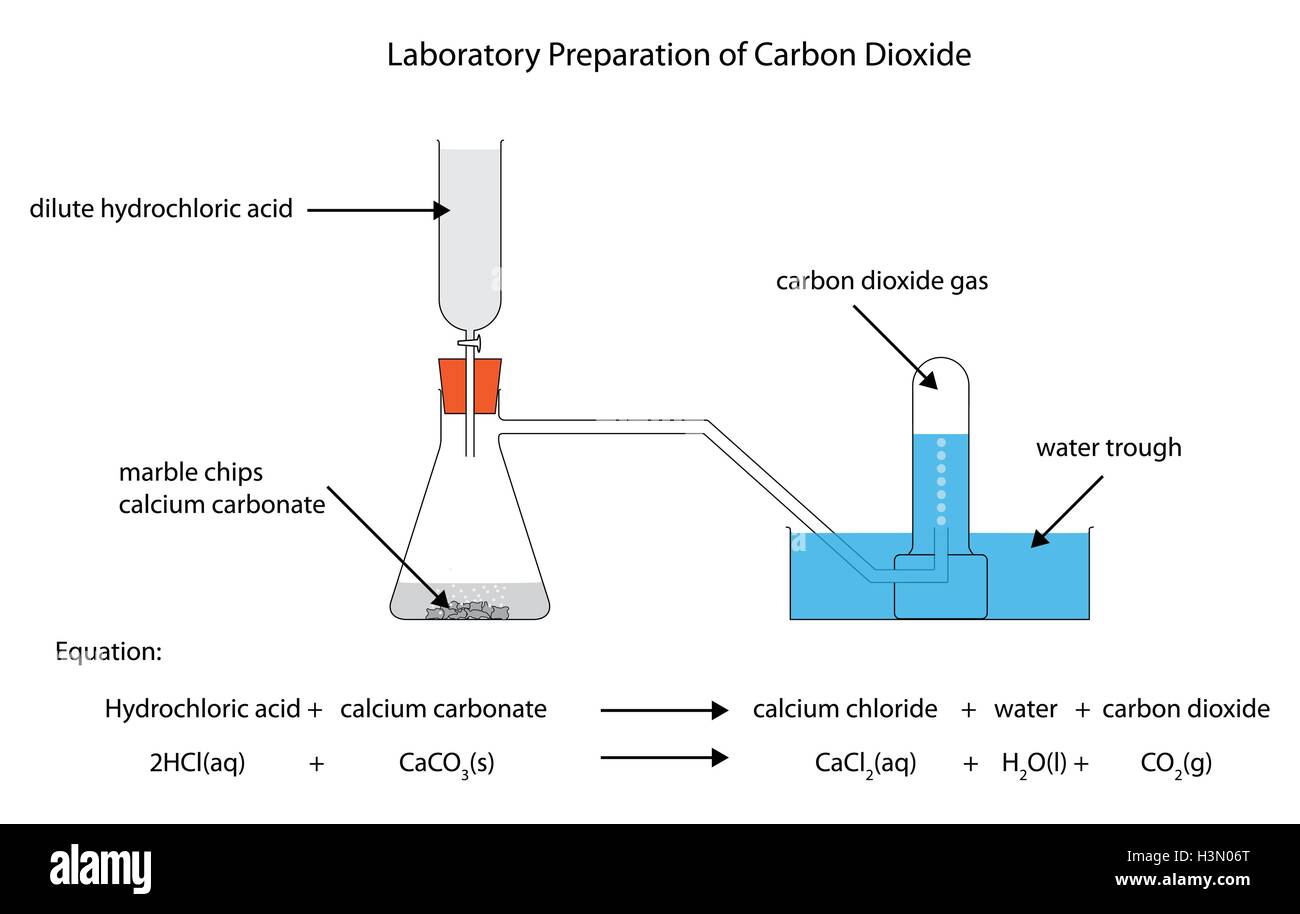
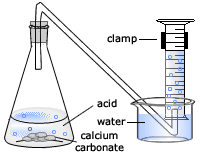
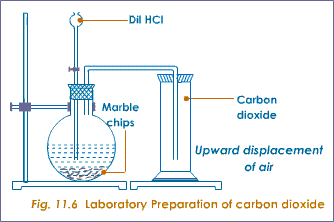
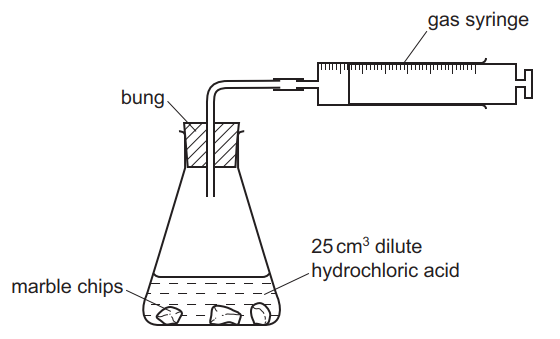
Marble chips and hydrochloric acid. Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim. A tube to connect the conical flask to the measuring cylinder. Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas. This is due to the collision theory.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide. The rate of this reaction can be changed by changing the size of the marble chips. A stand to hold up the measuring cylinder. Finding the rate of reaction of marble chips and hydrochloric acid changing the surface area.
This process is based on random particle movement. Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place. Finding the rate of reaction of marble chips and hydrochloric acid changing the surface area. A conical flask contains the marble chips hydrochloric acid and the water that will make the reaction.
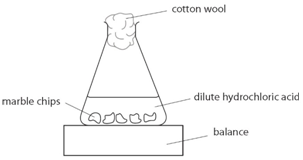
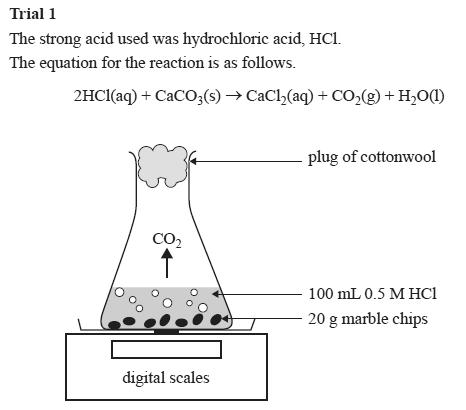
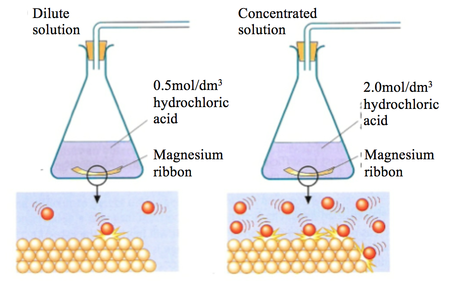
Caco3 2hcl h2o co2 this is the reaction we will be investigating. I predict the higher the concentration of hydrochloric acid faster the reaction rate and more carbon dioxide will be produced as the time increases. In high concentrated acid the particles of the marble chip will move faster due to the more collisions between the particles of marble chips and the acid. Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
The variables that i shall be changing will be the concentration of hydrochloric acid and water. An investigation into how changing one variable influences the rate of reaction between marble chips and dilute hydrochloric acid planning section when dilute hydrochloric acid reacts with marble chips the following reactions occurs. With the equation caco3 2hcl cacl2 h2o co2 hypotheses a reaction occurs when particles collide.