Marble Chips And Nitric Acid Experiment
The chemical equation that is going to be followed throughout the experiment will be.
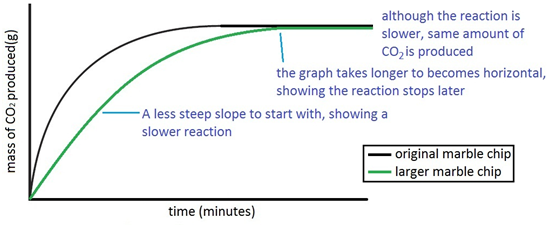
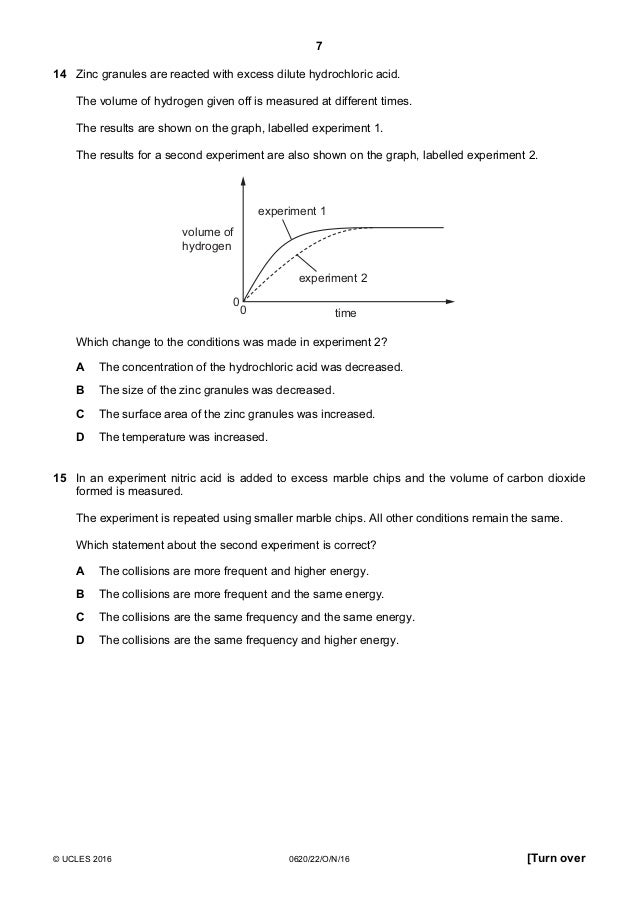
Marble chips and nitric acid experiment. 15 in an experiment nitric acid is added to excess marble chips and the volume of carbon dioxide formed is measured. An outline of an experiment that could be used to find the time and hence rate of reaction of marble chips and hydrochloric acid. The rate of this reaction can be changed by changing the size of the marble chips. Aim the experiment will be on the reaction of nitric acid and marble chips.
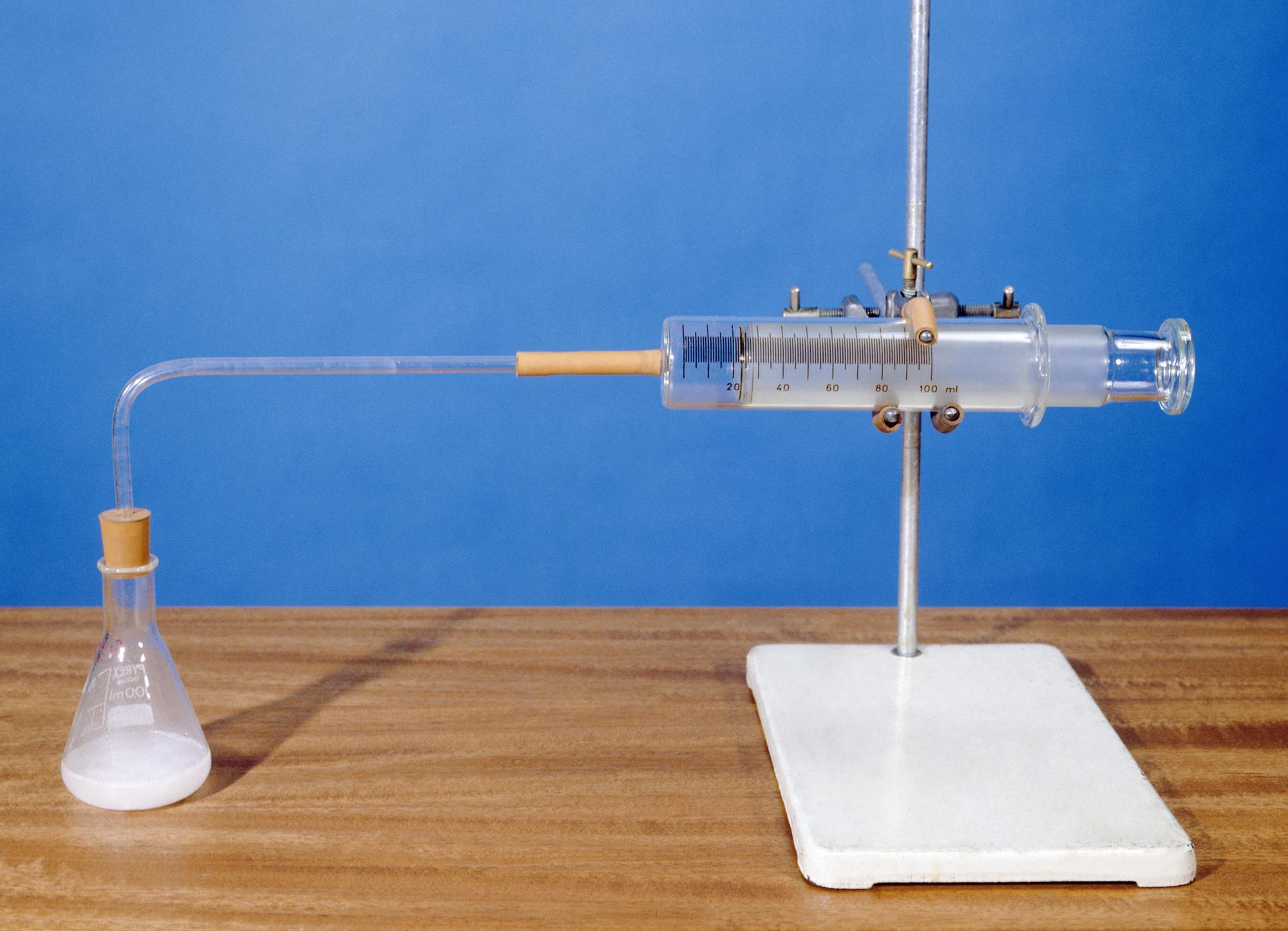
Marble is especially sensitive to the degrading by acidic chemicals also to weathering. Place 40cm 3 of hydrochloric acid in an conical flask. Conical flask glass jam jars measuring cylinder stop clock watch with seconds stop watch app direct reading balance dilute hydrochloric acid cotton wool marble chips method. One for concentrated hno3 has no2 nasty nitrogen dioxide as a product while the other for dilute hno3 has not.
Acid rain contains carbonic acid nitric acid and sulphuric acid co2 no2 and so2. This was done as the first trial showed that in the first 2 minutes the. The reason i think it would vary is that you get different reactions when one puts pure copper in either concentrated or dilute nitric acid. Calcium chloride solution is also formed.
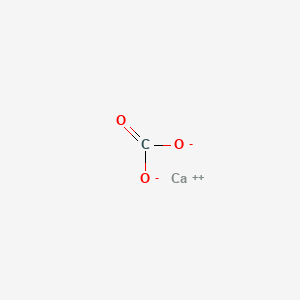
My guess is that the answer would vary depending on the concentration of the nitric acid. The marble chip is calcium carbonate caco3. The experiment is repeated using smaller marble chips. 3m hydrochloric acid with large marble chips 1 5m hydrochloric acid with small marble chips 3m hydrochloric acid with small marble chips a reading was taken initially every 15 seconds for a minute and then every 30 seconds for the next 4 minutes.
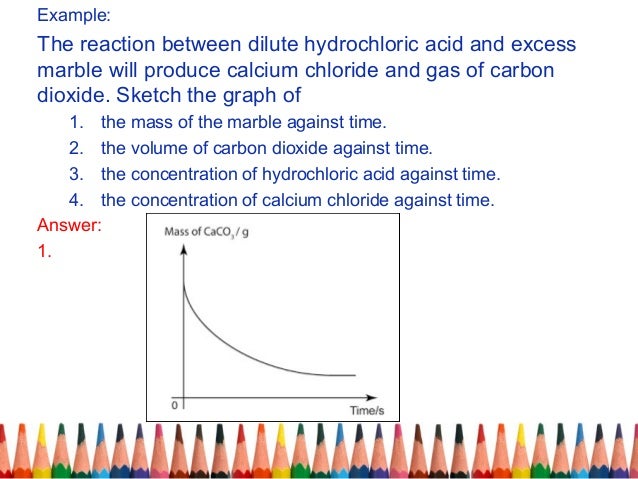
Acid rain is one of the top degradation agents for marble artefacts around the world. Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas. To investigate the effect of concentration on the reaction between marble chips and hydrochloric acid materials. Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas.
A the collisions are more frequent and higher energy. Using the apparatus shown the change in mass of carbon dioxide can be measure with time. Which statement about the second experiment is correct.